How to identify cell contamination?
Phenomenon 1: The cell culture medium becomes turbid, usually showing a yellow liquid like rice soup, which is mostly a sign of bacterial contamination
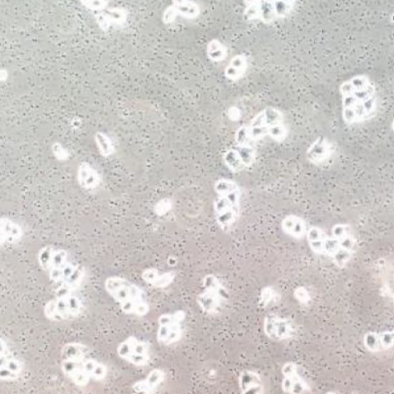
Illustration | Bacterial contamination
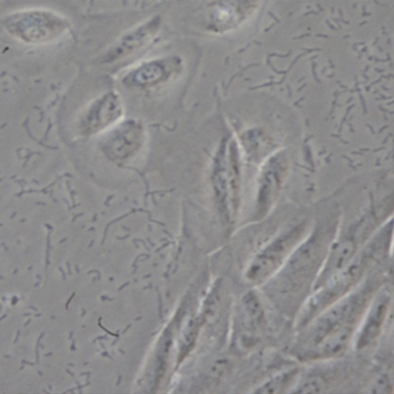
Illustration | Bacillus contamination
Phenomenon 2: There are active black spots in the culture medium, which is usually a sign of contamination by black gum worms.
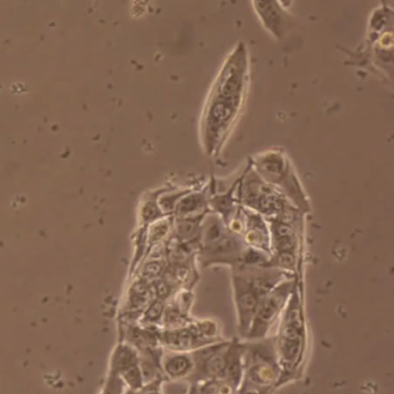
Illustration | Black glue bug
Phenomenon 3: Although the culture medium is clear, floating white hairy substances can be seen, which may be caused by fungal infection.

Illustration | Fungal contamination
Treatment method: If the cells are contaminated, it is recommended to discard the contaminated culture immediately and clean the incubator thoroughly with alcohol cotton balls, followed by half an hour of ultraviolet disinfection to prevent secondary contamination.
In addition, beginners can add penicillin and streptomycin to the culture medium during the culture process to reduce the risk of contamination.

GinoBio | Penicillin-Streptomycin Solution (100x)
During cell culture, small black spots appear around cells and on the cell surface. What should I do?
Phenomenon: If the small black spots are found to be immobile, it may be cell debris or mycoplasma contamination.
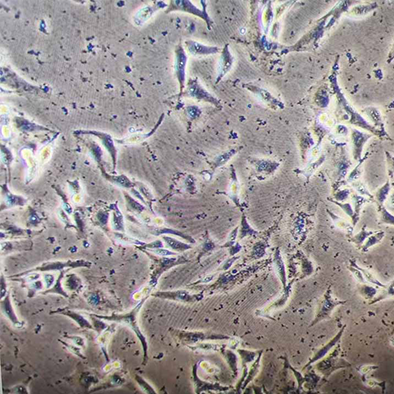
Illustration | Cell fragments
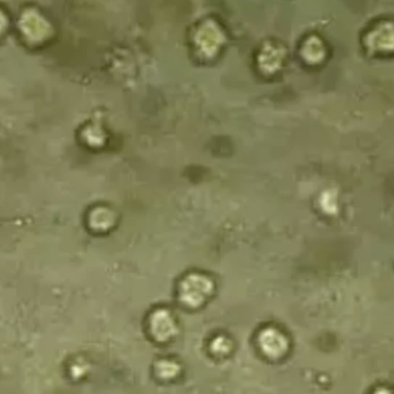
Diagram | Mycoplasma contamination
Treatment method:
For suspended cells: collect the supernatant, centrifuge at low speed (500~600rpm/min, 5~6min), and then replace with a new culture bottle.
For adherent cells: rinse with PBS 2~3 times, tap the culture bottle to remove loose debris, and then discard the PBS. Next, use a low concentration of trypsin (such as 0.05%) to digest for about 1 minute to remove the debris in the gap, and then digest and culture normally. If mycoplasma contamination is suspected, it is recommended to test, and cells with positive results should be processed and discarded as soon as possible. For precious cells, you can try to use mycoplasma removers (such as tylosin, etc.) and culture them separately from other cells.
Prevention method: Master the best time for cell passage to avoid excessive cell growth; control the digestion time to prevent excessive digestion from generating debris; reduce the number of freeze-thaw times of serum and other reagents; adjust the pH of the culture medium to the optimal state; ensure the water quality and cleanliness of utensils.

Genom Bio | PBS Buffer
There are many dead cells on the cell surface, which remain during subculture. How to deal with it?
Phenomenon: There are many dead cell clusters attached to the surface of adherent cells.
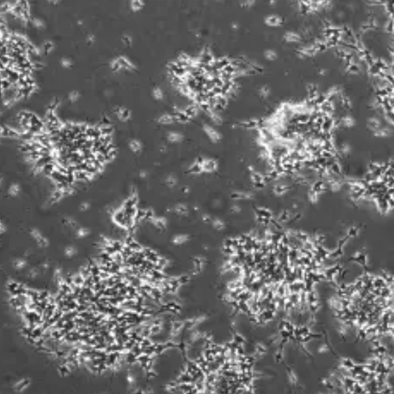
Illustration | Some dead cell clusters on the cell surface
Treatment method: After many practices, it has been proven that the effective method is to rinse twice with PBS, then add trypsin, shake gently and immediately pour out, rinse again with PBS, and then digest normally. Since dead cells have weak adhesion, they will fall off after the first addition of trypsin, and the cells digested for the second time are more active. After 2 to 3 passages, the cell state usually recovers well.
Genom Bio | 0.25% Pancreatin (Contains Phenol Red)
If the cytoplasm is loose and the cell state is poor, how to improve it?
Treatment method 1: Replace or increase the serum concentration to 15% to 20%, and then change it back to the normal 10% concentration after 48 hours of culture. Long-term use of high-concentration serum may be toxic to cells.
Treatment method 2: In addition to adjusting the serum concentration, you can also increase the cell density and transfer from the culture bottle to a six-well plate or a twelve-well plate. High-density culture can promote the growth of tumor cells through paracrine pathways.
Preventive measures: The main reasons for loose cytoplasm and aging are too many passages or too long trypsin digestion time. Control the number of passages and digestion time to avoid poor cell state and loose cytoplasm due to excessive digestion.
Cell passage
According to the cell growth characteristics, passage can be divided into two types: suspension cell passage and adherent cell passage. For suspension cells, you can add an equal amount of fresh culture medium and directly blow and disperse the passage, or replace the new culture medium after centrifugation and then blow and disperse the passage; adherent cells are passaged by digestion. The following are some precautions in passage culture.
Image
How to use trypsin when passaging adherent cells?
The trypsin-EDTA concentration used is usually 0.25% trypsin-0.53 mM EDTA.2Na or 0.05% trypsin-0.02 mM EDTA.2Na.
If the digestion solution concentration is too high, it will lead to an increase in cell debris and black residue in the culture medium. It is recommended to use 0.05% trypsin for routine cell passaging; for cells that are difficult to digest, 0.25% trypsin can be used for digestion. When the cell density exceeds 80%, a step-by-step digestion method can be used.
Trypsin should be stored at –20°C and thawed at 4°C. After opening the bottle for the first time, it is recommended to dispense it into sterile test tubes to avoid repeated freezing and thawing to maintain trypsin activity and reduce the chance of contamination.
How to control the trypsin digestion time?
Excessive trypsin digestion will lead to an increase in cell debris and black residue, large pieces of cells will fall off, and seriously affect cell activity. If the digestion is insufficient, the cells will be difficult to fall off the bottle wall, and repeated blowing may damage the cells. Therefore, it is particularly important to control the trypsin digestion time.
Different cells have different sensitivities to the digestion solution, and the trypsin digestion time is also different. For newly purchased cells, it is recommended to first use low-concentration trypsin to explore the digestion time, observe whether the cells become round under a microscope every 1 minute, and record the optimal digestion time for reference next time.
How to deal with the cell clumping phenomenon?
Suspended cells tend to clump at high density, and clumping cells are prone to death and affect the growth of surrounding cells. Larger cell clusters can be removed through a sieve, or the cell suspension can be collected into a 15mL centrifuge tube, left to stand for 20 minutes, and the upper cell supernatant can be carefully taken to continue culturing (this method can only remove some larger cell clusters).
Is it normal for vacuoles to appear in the cells?
Some cells themselves have certain vacuoles, such as HepG2, Ishikawa and other drug-resistant strains, which is a normal phenomenon. If only a few cells have very few vacuoles, it may be that the cells are in poor condition. The cell state can be improved by adjusting the serum concentration, controlling the digestion and passage time.
If most cells have vacuoles and the number is large, it may be due to aging caused by a high cell generation, and cells of early generations should be replaced.
What causes slow cell growth?
· Overdigestion
· Too close passage
· Malnutrition
· Poor cell state or aging
· Cell contamination
Reasons and solutions for cell non-adhesion
Trypsin treatment time: Insufficient digestion will cause cells to clump, and excessive digestion will easily damage cell membrane proteins, making cells loosely adherent, and in severe cases may cause cell death. It is recommended to shorten the trypsin digestion time or reduce the trypsin concentration.
Lack of adhesion factors: Serum contains factors that promote cell adhesion. The lack of such factors in serum-free culture medium will reduce the adhesion effect. The solution is to replace the serum.
Contamination: Contamination by mycoplasma or bacteria will cause poor cell adhesion. The culture should be separated, mycoplasma detected, and the incubator cleaned. If contamination is confirmed, the contaminated culture should be discarded.
Improper preparation or storage of culture medium: Too high or too low pH value of culture medium, improper aseptic operation, etc. will affect cell adhesion.
Cell freezing and recovery
Cell freezing and recovery are key steps to maintain the long-term stability of cell lines and preserve important cell resources. The following are some key points and precautions for cell freezing and recovery:
Preparation before cell freezing
Cell status: Ensure that the cells are in good condition, free of contamination and growing vigorously, and the cell density is generally controlled between 70%-90%. It is recommended to freeze 1-2 days after subculturing, when the cell viability is higher.
Selection of culture medium: Use culture medium containing 10% FBS (fetal bovine serum) and DMSO (dimethyl sulfoxide) as freezing medium. Usually, the formula of freezing medium is 90% complete culture medium + 10% DMSO, or 80% complete culture medium + 20% FBS + 10% DMSO.
Cell collection: For adherent cells, collect cells after trypsin digestion, and collect suspended cells directly by centrifugation. It is recommended to discard the supernatant after centrifugation at 1000 rpm for 5 minutes and add an appropriate amount of freezing solution to resuspend the cells.

Genom Biotech | Fetal Bovine Serum (Superior Grade)
Cryopreservation steps
Dispensing: Dispense the suspended cell solution into cryopreservation tubes in 1-1.5 mL volumes, ensuring that the tube mouth is well sealed.
Cooling rate: During the cryopreservation process, the cooling rate is crucial to the survival rate of cells. Usually, the programmed cooling method is used to refrigerate the cells at 4°C for 10-15 minutes, then transfer to -20°C for 30 minutes, and then transfer to -80°C overnight. Finally, the cryopreservation tubes are moved to a liquid nitrogen tank for long-term storage. By slowly cooling, ice crystals are prevented from forming in the cells and cell damage is reduced.
Genom Bio | Serum-free cell cryopreservation medium
Cell recovery
Recovery speed: Thaw as quickly as possible during recovery to reduce damage to cells by ice crystals. Usually, after taking the cryovial out of the liquid nitrogen tank, immediately place it in a 37°C water bath and shake it quickly until the ice is completely melted.
Removal of DMSO: After cell recovery, DMSO needs to be removed as soon as possible. Transfer the thawed cell solution quickly to 10mL of pre-warmed culture medium containing 10% FBS, centrifuge at 1000rpm for 5 minutes, discard the supernatant, add fresh culture medium to suspend the cells, and inoculate them into culture bottles.
Culture observation: The recovered cells should be placed in a 37°C, 5% CO2 incubator for static culture. After 24 hours, replace the culture medium to remove dead cells and residual DMSO. Subsequently, cell passage and culture are performed as usual.
Common problems in cell freezing and recovery
Low viability of frozen cells: This may be related to improper formulation of the freezing solution, too fast cooling rate, or improper recovery operation. It is recommended to optimize the freezing conditions to ensure that each step is strictly carried out in accordance with the standards. Poor cell adhesion or slow proliferation after recovery: This may be caused by DMSO residue, poor cell state or cell damage. DMSO can be removed by multiple washings, the composition of the culture medium can be adjusted, or the serum concentration can be increased in the early stage of recovery to promote cell recovery.
Stability of long-term storage of frozen cells: Long-term storage of cells in liquid nitrogen can maintain a high survival rate, but long-term storage at -80°C may affect cell viability. It is recommended to store them in liquid nitrogen tanks as much as possible after freezing.
The above is a detailed guide to cell culture, passaging, freezing and recovery. I hope it will be helpful for your cell experiments. If you encounter other problems or need more advice, please feel free to contact Jinuo Bio!
| Part Number | Product Name | Specification |
|---|---|---|
GNM15140-1 | Penicillin-streptomycin solution (100×) | 100ml |
GNM20012-5 | PBS buffer pH 7.2 | 500ml |
GNM15050-1 | 0.25% Pancreatin with Phenol Red | 100ml |
GNMFBCP5 | Fetal bovine serum (superior grade) | 500ml |
GNMA1042 | Serum-free cell freezing medium | 100ml |